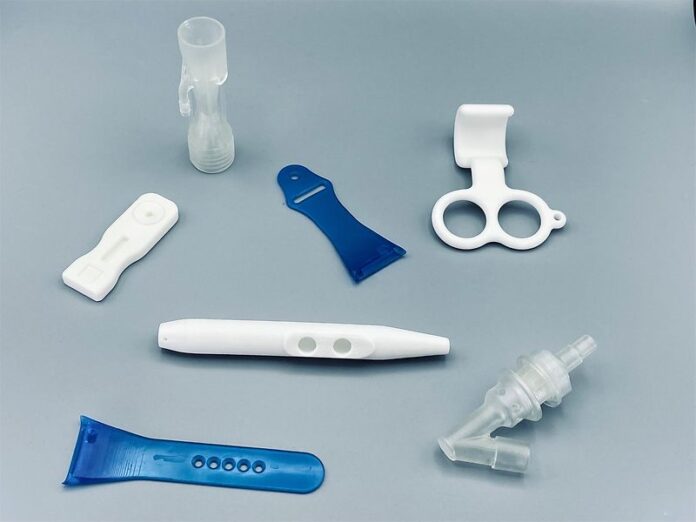
Henkel AG & Co., Düsseldorf, Germany, has announced that its Concord laboratory and Dixon production facilities in California, which are dedicated to developing and manufacturing resins for 3D printing, have achieved ISO 13485:2016 certification.
ISO 13485 is an international quality standard that enables medical device manufacturers to specify and implement process and production controls, in addition to providing them with documentation and traceability. In Henkel’s case, it covers the design and manufacturing of biocompatible resins and other materials used to produce non-implantable medical devices.
The areas of operation that are reviewed for compliance include, design control, process and production controls, change management control, product traceability and risk management.
For more information, visit www.LoctiteAM.com.